Comparative Analysis of Clonal Evolution in Right- and Left-Sided Colon and Rectal Cancer
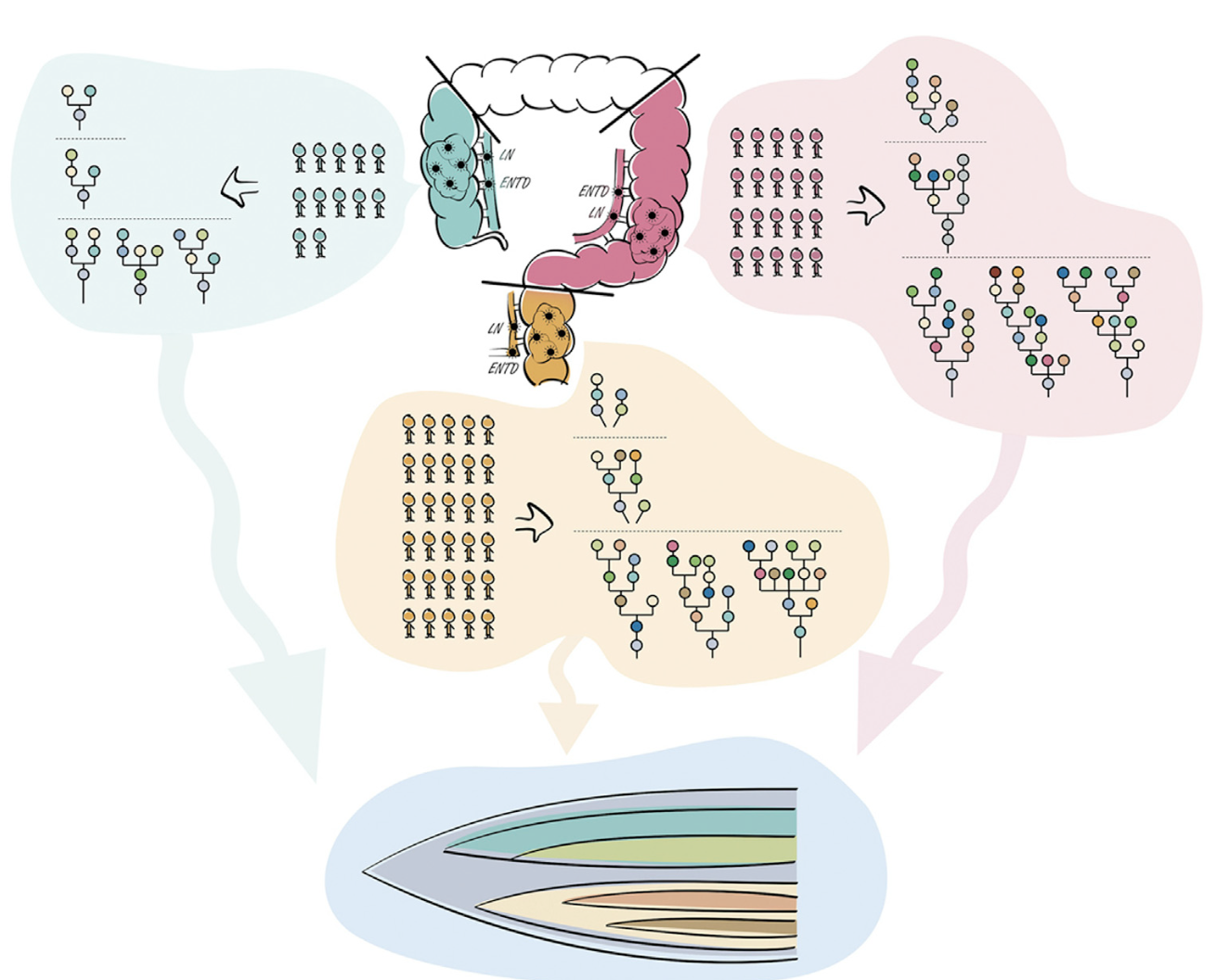
Tumor evolution is a dynamic and complex process, with variations in growth patterns significantly influencing disease progression and therapeutic outcomes. In our latest study, published in iScience, we utilized high-depth whole-exome sequencing (WES) of 206 tumor regions from 68 colorectal cancer (CRC) patients to unravel intratumor heterogeneity (ITH) and clonal evolution across different tumor locations—right-sided colon cancer (RCC), left-sided colon cancer (LCC), and rectal cancer (RC). By integrating evolutionary insights with genetic evidence, we analyzed metastasis pathways involving lymph nodes (LN) and extranodal tumor deposits (ENTDs), revealing distinct patterns of tumor development.
Key Findings
-
Distinct Evolutionary Pathways Between RCC, LCC, and RC:
Our study found higher complexity and divergence in LCC and RC tumors compared to RCC. Through phylogenetic tree analysis, we observed more branched evolution in LCC and RC, suggesting increased genetic variability and adaptability. -
Tumor Metastasis and Polyclonality:
While LN and ENTDs were previously assumed to share common origins, our findings demonstrate that both metastases originated from different clones. Moreover, ENTD evolved later than LN metastases, providing an essential distinction for future TNM staging classifications. -
Genetic Signatures and Key Driver Events:
Mutational analysis revealed higher intratumor heterogeneity (ITH) in RC, with fewer clonal mutations compared to RCC. Key tumor-driver genes, including APC, TP53, and KRAS, were confirmed as early events in tumor initiation, while SMAD4 alterations were predominantly detected in metastatic lesions. -
Parallel Evolution and Tumor Progression:
By detecting mirrored subclonal allelic imbalance (MSAI) events, we identified cases of parallel evolution, highlighting convergent mutational pathways that influence CRC progression.
Reflections
Morphogenesis is a fundamental biological process, with symmetry forming the basis of most structural development. However, asymmetry—especially subtle variations in bilaterally symmetrical organs—often emerges. In disease pathology, such asymmetry can amplify critical differences, providing a unique lens through which tumor progression and abnormal growth can be examined.
Many past studies have demonstrated that asymmetric tumor development can be an invaluable tool in identifying key factors that drive disease onset and progression, particularly within similar genetic backgrounds. By systematically investigating the evolutionary divergence among CRC subtypes and mapping out genetic variations linked to tumor asymmetry, we take a crucial step toward refining precision medicine approaches.
This study was led by Santasree Banerjee, a key member of our disease research group in Qingdao, and exemplifies our commitment to unraveling the genomic complexities of cancer evolution through collaborative, high-resolution sequencing strategies.
The full text of this study can be accessed online at iScience.