Cellular and Molecular Insights into Fibrosis and Resolution in the Bleomycin-Induced Pulmonary Fibrosis Mouse Model
Pulmonary fibrosis is a chronic lung condition with limited treatment options and significant mortality rates. To better understand this condition, we utilized the widely accepted bleomycin-induced pulmonary fibrosis mouse model and applied Stereo-seq spatial transcriptomics technology to investigate the cellular and molecular changes throughout fibrosis and resolution processes. Our study provides valuable insights into the progression of fibrosis, cellular dynamics, and potential markers of fibrosis resolution.
Key Findings
-
Spatiotemporal Transcriptome Atlas Construction:
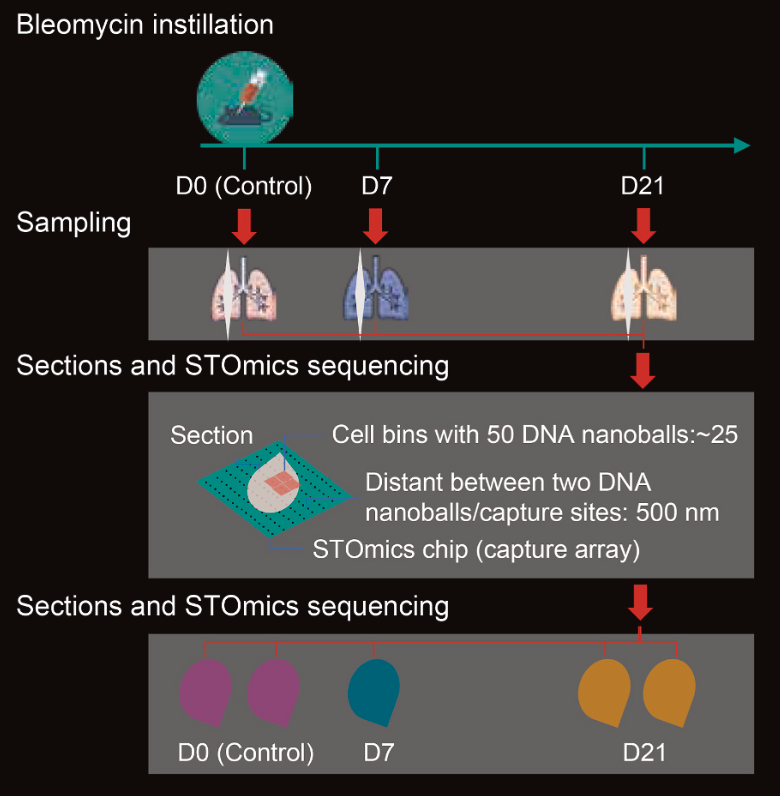
We generated a spatiotemporal transcriptome atlas of mouse lungs at three critical time points: baseline (control), acute fibrosis (7 days after bleomycin injection), and resolution phase (21 days after injection). This dataset represents the first spatial transcriptomics analysis of the bleomycin-induced pulmonary fibrosis mouse model. - Changes in Cellular Composition and Dynamics:
Our analysis highlighted significant changes in key lung cell types during fibrosis:- Alveolar type II (AT2) cells, responsible for lung repair, decreased dramatically in numbers during fibrosis.
- Fibroblasts showed a steady increase, particularly the myofibroblast subtype, which is closely linked to extracellular matrix accumulation and fibrosis progression.
- Macrophages and other immune cells increased transiently during the acute fibrosis stage, reflecting their involvement in inflammatory responses.
-
Gene Expression and Resolution Mechanisms:
We identified differential expression of genes related to fibrosis and self-resolving processes. For example, fibrosis markers such as Spp1 and Col1a1 were upregulated during acute fibrosis, while recovery-phase genes like Prkca, which is associated with inflammation alleviation, were observed during the resolution phase. - Novel Insights into Cellular Interactions:
Using cell-cell communication analysis, we uncovered increased interactions between fibroblasts and macrophages during fibrosis, mediated by key signaling molecules such as COL1A1 and APP. These interactions gradually shifted in the resolution phase, suggesting pathways that may play critical roles in fibrosis recovery.
Reflections
At the time Stereo-seq technology was introduced, I was setting up the Beijing branch of our institute. While building the experimental platform, we tested various samples, including the mouse lung tissues used in this project. This research was initiated based on a recommendation from Dr. Zhang of Tongji Hospital, who suggested exploring the bleomycin-induced pulmonary fibrosis model in mice. The experimental design was straightforward: sampling the lungs of model mice at different stages and performing spatial transcriptomics analysis. We analyzed three spatial transcriptomics sections in total.
While the data quality was promising, it was not perfect, as it was initially intended as test data. Nonetheless, I proposed writing this paper to leverage the available dataset. For pulmonary fibrosis research, the bleomycin model has its limitations in terms of acceptance, and a more comprehensive design incorporating additional time points, experimental conditions, and follow-up validations could potentially produce a stronger paper. Despite these constraints, we presented findings on lung cell types, molecular changes during fibrosis progression, and cellular processes during fibrosis resolution. Enhancing the model design and adding further experimental validations in future studies could result in an even more impactful publication.
The full text of this study can be accessed online at Heliyon.